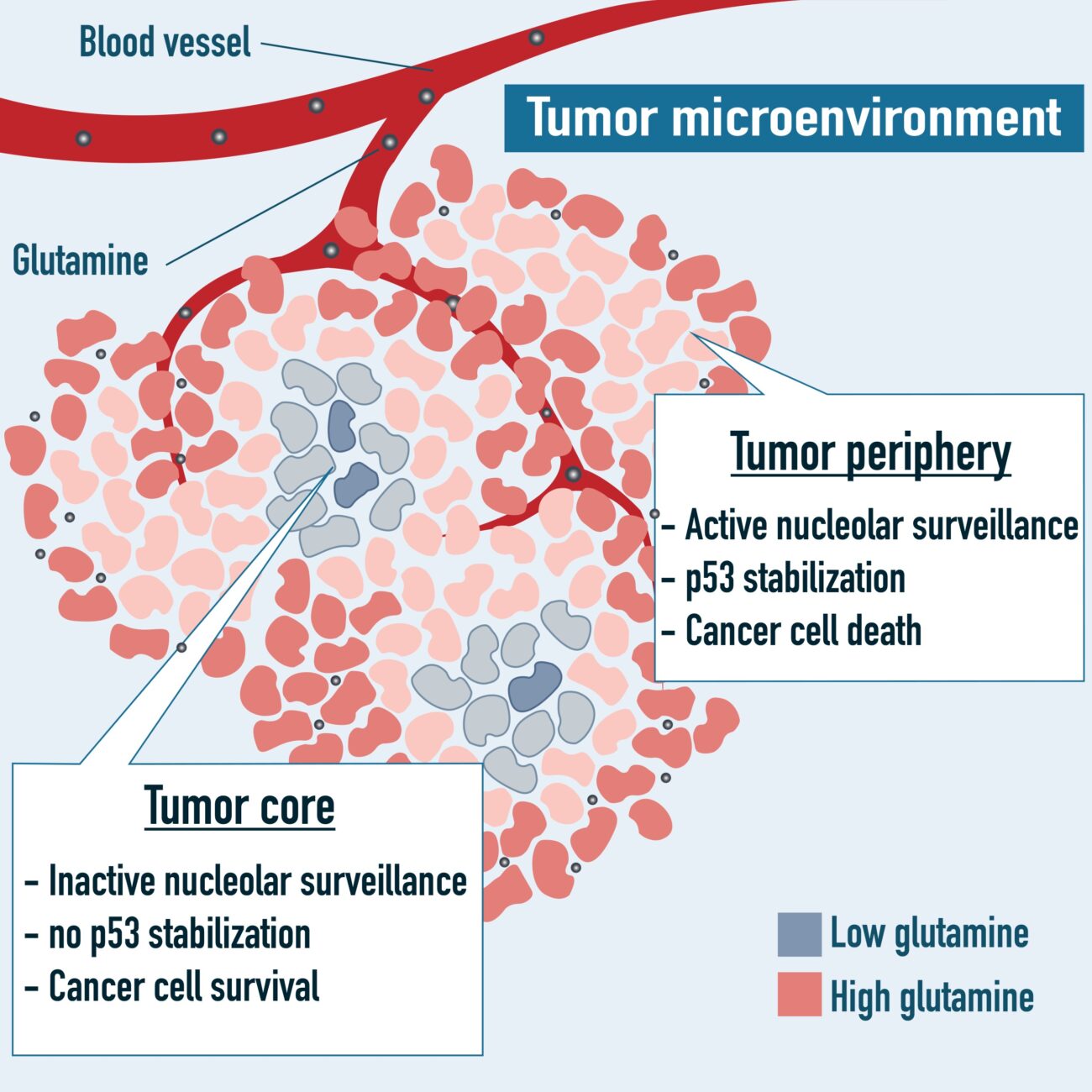
Nucleolar surveillance is a p53-dependent anti-tumoral pathway activated upon ribosome biogenesis dysfunction. It had remained unclear why liquid tumours respond better to ribosome biogenesis inhibitors than solid ones. In collaboration with the team of Tsuyoshi Osawa (U. Tokyo), we have revealed the importance of the intra tumoral concentration of the amino-acid glutamine for nucleolar surveillance activation. The study was published in Nature Communications during the summer of 2022.

Glutamine deficiency in solid tumor cells confers resistance to ribosomal RNA synthesis inhibitors
Ribosome biogenesis is an energetically expensive program that is dictated by nutrient availability. Here we report that nutrient deprivation severely impairs precursor ribosomal RNA (pre-rRNA) processing and leads to the accumulation of unprocessed rRNAs. Upon nutrient restoration, pre-rRNAs stored under starvation are processed into mature rRNAs that are utilized for ribosome biogenesis. Failure to accumulate pre-rRNAs under nutrient stress leads to perturbed ribosome assembly upon nutrient restoration and subsequent apoptosis via uL5/uL18-mediated activation of p53. Restoration of glutamine alone activates p53 by triggering uL5/uL18 translation. Induction of uL5/uL18 protein synthesis by glutamine is dependent on the translation factor eukaryotic elongation factor 2 (eEF2), which is in turn dependent on Raf/MEK/ERK signaling. Depriving cells of glutamine prevents the activation of p53 by rRNA synthesis inhibitors. Our data reveals a mechanism that tumor cells can exploit to suppress p53-mediated apoptosis during fluctuations in environmental nutrient availability.